


Harness the healing power of SuperSaturated Oxygen (SSO2) Therapy to reduce the risk of heart failure
The first FDA-approved, catheter-based therapy to safely and effectively reduce infarct size in randomized control trials.2,3
Delivers localized hyperoxemic levels of oxygen to treat ischemic myocardium, without impacting door-to-balloon time.
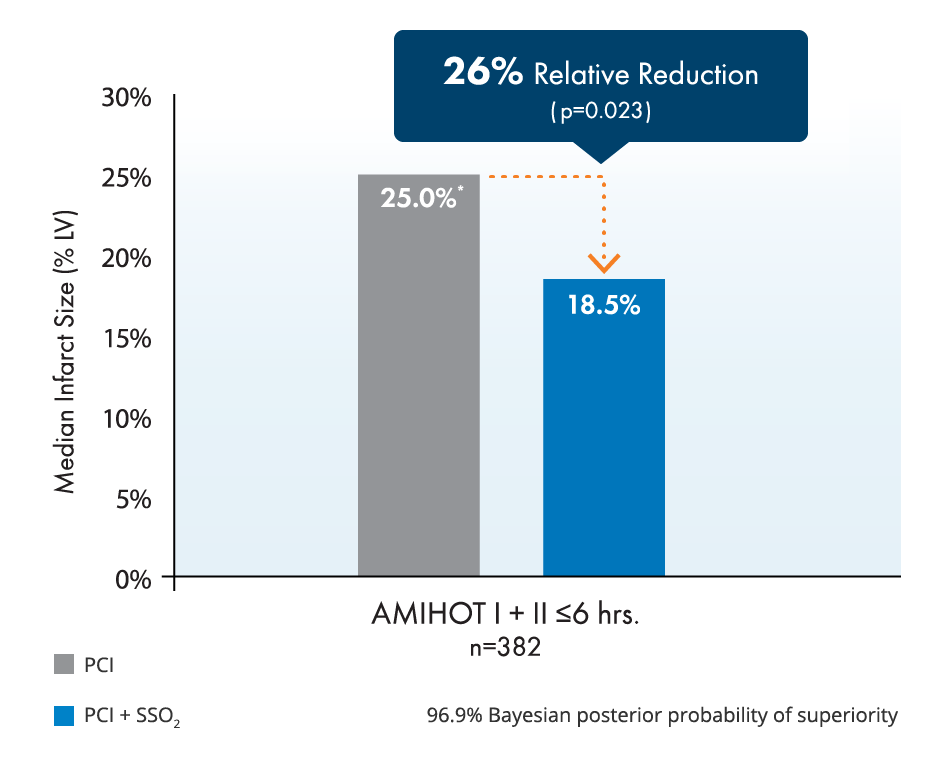
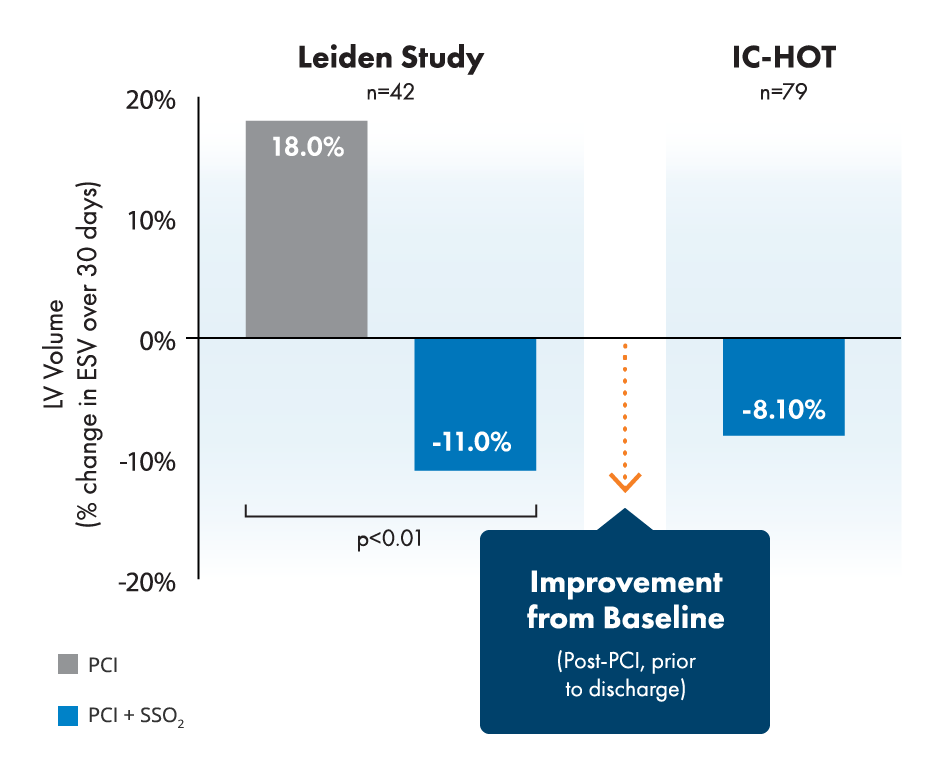
BrochureStudies show 26% relative infarct size reduction and improvement of left ventricular function in LAD STEMI.2,3,4,5
PublicationTreatment with SSO2 was associated with a lower 1-year rate of all-cause death or new-onset heart failure (HF) of hospitalization for HF (0.0% vs. 12.3%, p = .001).6
Publication
SuperSaturated Oxygen (SSO2) Therapy has been shown to reduce endothelial swelling and restore microvascular flow, leading to reductions in infarct size.7*
90% of myocardial blood flow is supplied by the microvasculature.8
Microvascular obstruction is strongly associated with mortality and heart failure hospitalization within 1 year.9
A 26% relative reduction in infarct size has been correlated with relative reductions in both death and heart failure hospitalization of ~25% at 1 year.4
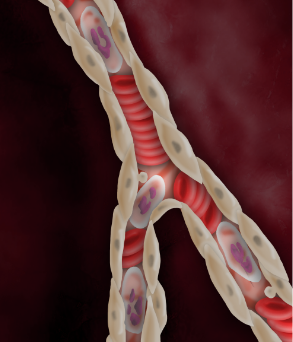
Despite successful PCI, endothelial edema occurs and restricts microvascular flow.
Despite successful PCI, endothelial edema occurs and restricts microvascular flow.
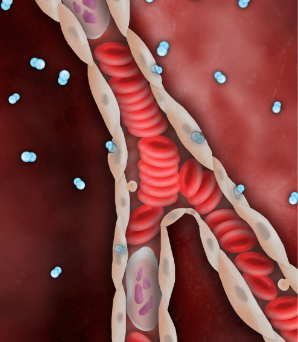
SSO2 Therapy delivers high levels of dissolved O2 (pO2 = 760-1000mmHg), via the plasma, even before flow is restored downstream.
SSO2 Therapy delivers high levels of dissolved O2 (pO2 = 760-1000mmHg), via the plasma, even before flow is restored downstream.
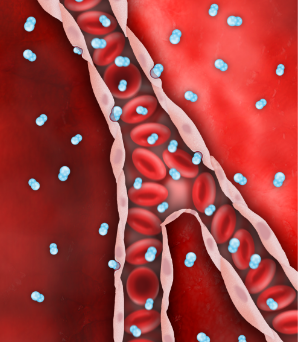
Endothelial edema is resolved, restoring capillary flow and reperfusing ischemic myocardium.
Endothelial edema is resolved, restoring capillary flow and reperfusing ischemic myocardium.
Please refer to IFU for full cautions, warnings and Indications for Use.
Caution: Federal law restricts this device to sale by or on the order of a physician.
Indications For Use: The TherOx DownStream System is indicated for the preparation and delivery of SuperSaturated Oxygen Therapy (SSO2 Therapy) to targeted ischemic regions perfused by the patient’s left anterior descending coronary artery immediately following revascularization by means of percutaneous coronary intervention (PCI) with stenting that has been completed within 6 hours after the onset of anterior acute myocardial infarction (AMI) symptoms caused by a left anterior descending artery infarct lesion.
© 2024 ZOLL Medical Corporation, Chelmsford, MA, USA. TherOx and ZOLL are trademarks or registered trademarks of ZOLL Medical Corporation in the United States and/or other countries.
